What is Cyclodextrin?
Cyclodextrins are crystalline, water soluble, cyclic, non-reducing, oligosaccharides built up from six, seven, or eight glucopyranose units. Three naturally occurring CDs are alpha Cyclodextrin, beta Cyclodextrin, and gamma Cyclodextrin. All the hydroxyl groups in Cyclodextrins are oriented to the outside of the ring while the glucosidic oxygen and two rings of the non-exchangeable hydrogen atoms are directed towards the interior of the cavity. This combination gives Cyclodextrins a hydrophobic inner cavity and a hydrophilic exterior. The hydrophobic internal cavity provides the capability to form inclusion complexes with a variety of "guest" hydrophobic molecules (e.g. aromatics, alcohols, halides, fatty acids, esters, etc.).
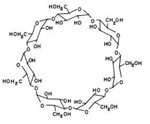
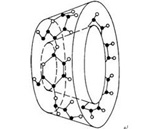
How does Cyclodextrin look like?
Cyclodextrins have a specific toroidal shape: a tight conical cylinder with a hydrophilic exterior (due to the presence of hydroxyl radicals) and a hydrophobic cavity of a specific size. Generally 6 - 8 sugar units.
 |
 |
What are the types of Cyclodextrin?
The most common cyclodextrins are alpha, beta, and gamma cyclodextrins having six (¦Á), seven (¦Â), or eight (¦Ã) anhydroglucose units in the ring structure. Among them, beta Cyclodextrin is mostly common used.
Different sizes lead to different physicochemical characteristics for the respective molecules:
Characteristics |
Alpha (¦Á) |
Beta (¦Â) |
Gamma (¦Ã) |
Number of glucose units |
6 |
7 |
8 |
Molecular weight |
972 |
1135 |
1297 |
Water solubility(g/100ml), 25¡ã C |
14.5 |
1.85 |
23.2 |
Cavity diameter (nm) |
0.57 |
0.78 |
0.95 |
Height of torus (nm) |
0.78 |
0.78 |
0.78 |
Chemically modified Cyclodextrins
Cyclodextrins can be modified by various procedures such as:
Substituting one or more hydrogen atoms in the primary and/or secondary hydroxyl groups (esters, ethers, glycosylcyclodextrin)
Substituting one or more primary and/or secondary hydroxyl groups
Chemically modified Cyclodextrins (CDM's) exhibit substantially increased aqueous solubility with concomitant retention of the inclusion complexing properties of the starting Cyclodextrin. They therefore appear to offer specific applications within various industries, such as foods, pharmaceuticals, personal care/cosmetics, diagnostics, etc. Hydroxyalkyl b Cyclodextrins such as Hydroxypropyl b Cyclodextrin are examples of those chemically modified Cyclodextrins.
How does Cyclodextrin work?
Cyclodextrin inclusion is a molecular phenomenon in which usually only one guest molecule interacts with the cavity of a Cyclodextrin molecule to become entrapped and form a stable association. Molecules or functional groups of molecules those are less hydrophilic than water, can be included in the Cyclodextrin cavity in the presence of water.
In order to become complex, the "guest molecules" should fit, at least partly, into the Cyclodextrin cavity. The cavity sizes as well as possible chemical modifications determine the affinity of Cyclodextrins to the various molecules. In the case of some low molecular weight molecules, more than one guest molecule may fit into the cavity. On the opposite, some high molecular weight molecules may bind more than one Cyclodextrin molecule. Therefore a 1 to1 molar ratio is not always achieved, which broadens the range of applications.
Cyclodextrins are able to form inclusion complexes with broad range hydrophobic molecules as poorly soluble drugs, rapidly deteriorating flavours, volatile fragrances, toxic pesticides or dangerous explosives, even gases; entrapping these substances in their inner cavities. For example, a-Cyclodextrin forms inclusion complexes with both aliphatic hydrocarbons and gases, such as carbon dioxide. B-Cyclodextrin typically forms complexes with small aromatic molecules. G-Cyclodextrin can accept more bulky compounds, including vitamin D2 or organic macro cycles.
This results in the physical and chemical characteristics of the guest molecules:
In the solid state:
The guest molecule is molecularly dispersed in the CD matrix, even with gaseous guest molecules.
The guest molecule is effectively protected against any type of reaction, except with CD's hydroxyls.
Sublimation and volatility are reduced to a very low level.
In aqueous solution:
Concentration of the poorly soluble guest molecule in the dissolved phase increases significantly.
Reactivity of the guest molecule decreases in most cases.
What does Cyclodextrin do?
1.Solubilization:
Enhancement of water solubility of lipophilics
Change of rheological Properties Increase of bioavailability
2.Stabilization
Of an active ingredient
Of an emulsion
Of a volatile compound
Against UV light
Against temperature
Against Oxidation
Against hydrolysis
3.Reduction of
Bad taste
Unpleasant odour
Skin irritation
Hygroscopicity
4.Controlled release of actives
5.Selective extraction
Where is Cyclodextrin used?
Ink Industry:
Water soluble dyes in water based systems for ink jet printers
Water resistance - fixing characteristics in ink jet printers
Water resistance in thermal ink jet printing
Aqueous composition/wettability in writing ustencils
Greater florescent yield in highlighter pen
Fragrance / Flavour:
Controlled release (ex : Chewing-gum flavor).
Complexation of perfumes/oils
Food:
Selective extraction of Naringen& limonen from citrus juice
Selective extraction of caffeine from tea/coffee.
Selective extraction of pigments which are precursors of enzymic browning of fruit juices.
Complexation of triglycerides in vegetable oils with a high content of polyunsaturated fatty acids to improve stability.
Cosmetics:
Reduced irritancy of fragrances in shampoos
Controlled release of actives (ex : fragrance in body lotion)
Protection of actives against oxidization
Skin treatment of acne, through complexation of sebum.
Foot deodorants
Increased stability of antiplaque toothpaste
Higher concentration of lipophilic substances in aqueous emulsions (ex: face lotions)
Masking unpleasant odour of a volatile compound (ex: mercaptan in permanent wave product / self tanning products).